페이지 정보
Low-energy catalytic methanolysis of poly(ethyleneterephthalate), publ…
본문

Abstract
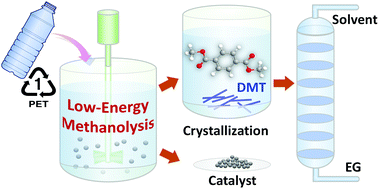
Methanolysis is a chemical pathway for depolymerizing post-consumer PET plastic waste into monomeric feedstock, which can be utilized as a starting component to produce polymer materials with the same quality as the original polymer or other valuable products. In general, conventional methanolysis is carried out at a high reaction temperature under high pressure, demanding high capital and operating costs and in turn leading to an adverse effect on the environment from CO2 emissions. In this study, we developed a low-energy catalytic route for methanolysis to convert PET resin to dimethyl terephthalate (DMT). Potassium carbonate (K2CO3), which is an inexpensive and nontoxic salt, was used as a catalyst, and the effects of cosolvents on the catalytic performance were investigated to develop a new decomposition pathway towards DMT at ambient temperature. Compared to existing methanolysis processes, the overall reaction rate of the proposed system was relatively slow and steady, but the PET resins were completely decomposed into monomers within 24 hours. Intriguingly, a high selectivity of DMT was obtained at a mild temperature range of 20–35 °C. The yield of DMT, obtained by methanolysis at 25 °C, was 93.1% as the molar ratios of methanol, dichloromethane and K2CO3 to PET repeating units were 50, 50, and 0.2, respectively. In this experimental setup, the initial molar ratio of moisture to PET repeating units was adjusted to be 0.4. 2-Hydroxyethyl methyl terephthalate and monomethyl terephthalate were the major by-products created during the process. It was demonstrated that the ideal conversion of PET into DMT could be achieved by controlling the moisture level. In addition to several analytical methods for product characterization, we performed a parametric study to probe the most likely reaction steps and to observe the reaction behaviour of PET methanolysis. Based on the proposed mechanism, a kinetic model was developed and compared with the experimental data to estimate kinetic parameters. In the reaction system, PET depolymerization apparently proceeded through two series of reaction steps, and the degradation of PET had a relatively low activation energy of 66.5 kJ mol−1, which was accountable for catalytic methanolysis of PET at ambient conditions.